Welcome to the Webinar on Pharmaceutical Chemistry! We are thrilled to have you join us for an in-depth exploration of the latest trends, breakthroughs and innovations in the pharmaceutical chemistry field. This event will bring together experts and professionals to share valuable insights that can shape the future of medicine and healthcare. Get ready to learn, network, and discover the possibilities within the pharmaceutical world.
Whether you are a student, a professional in the pharmaceutical industry, or simply curious about the science behind medications, this webinar is designed for you. Our expert speakers will share their insights, research findings, and practical applications in the field, providing you with valuable knowledge and networking opportunities.
Don’t miss out on this chance to expand your horizons and connect with like-minded individuals. We look forward to seeing you there!


The GlobalMeetx Pharmaceutical Chemistry Webinar is a premier online event designed to connect researchers, scientists, industry professionals, and students passionate about pharmaceutical chemistry. This webinar will provide a platform for sharing groundbreaking research, discussing emerging trends, and fostering collaboration within the global pharmaceutical community. Our expert speakers will cover a range of topics, including Drug Design and Development, Formulation Chemistry, Sustainable Chemistry in Drug Development.
Join us for a day of valuable discussions, expert sessions, and networking opportunities that will enhance your understanding of the pharmaceutical industry and its profound impact on public health.
This track focuses on the essential stages of developing new drugs, from initial discovery to market approval. Key topics include:
Target Identification and Validation – Discovering and validating targets for drug intervention.
Hit Discovery and Screening – Identifying potential drug candidates using advanced screening technologies.
Lead Optimization – Improving drug potency and pharmacokinetic properties through design and testing.
Preclinical Development – Assessing safety and efficacy in lab models before human trials.
Clinical Trials – Conducting Phase I-III trials to evaluate safety, dosage, and effectiveness.
Medicinal chemistry and drug design focus on the discovery and development of new therapeutic agents through the application of chemical principles. It involves the design, synthesis, and optimization of bioactive compounds to improve their potency, selectivity, and safety. This field combines organic chemistry, pharmacology, and biology to create molecules that interact with specific biological targets, such as proteins or enzymes, to treat diseases. By understanding structure-activity relationships (SAR), medicinal chemists can refine compounds to enhance their therapeutic effectiveness while minimizing side effects. The integration of computational modeling, high-throughput screening, and structure-based drug design has revolutionized drug discovery, enabling the development of more targeted and personalized therapies.
This track focuses on the innovative intersection of pharmaceutical formulations and nanotechnology in drug development. Key topics include:
Pharmaceutical Formulation Development – Designing optimal drug delivery systems for improved bioavailability, stability, and patient compliance.
Nanotechnology in Drug Delivery – Using nanomaterials to enhance drug solubility, targeted delivery, and precision medicine.
Nanoparticles for Targeted Therapy – Exploring the use of nanoparticles to deliver drugs directly to specific tissues or cells for more effective treatments.
Analytical techniques are crucial in ensuring the quality and safety of pharmaceutical products. Methods like chromatography (HPLC, GC), spectroscopy (UV-Vis, IR, NMR), and mass spectrometry are used to identify, quantify, and assess the purity of drugs. These techniques help detect impurities, confirm chemical structures, and monitor drug performance, ensuring compliance with regulatory standards and effective treatment outcomes.
Analytical techniques are crucial in ensuring the quality and safety of pharmaceutical products. Methods like chromatography (HPLC, GC), spectroscopy (UV-Vis, IR, NMR), and mass spectrometry are used to identify, quantify, and assess the purity of drugs. These techniques help detect impurities, confirm chemical structures, and monitor drug performance, ensuring compliance with regulatory standards and effective treatment outcomes.
Clinical research and trials are critical to evaluating the safety, efficacy, and overall therapeutic value of new drugs and treatments. These studies are conducted in phases, starting with small-scale trials in healthy volunteers (Phase I) to assess safety and dosage, followed by larger trials in patients (Phase II and III) to evaluate effectiveness, side effects, and optimal dosing. Clinical trials are tightly regulated and must adhere to ethical standards to ensure participant safety and data integrity. The results of these trials provide the necessary evidence for regulatory approval from agencies like the FDA or EMA. Successful clinical research ultimately leads to new treatments, improving patient outcomes and advancing medical science.
This track explores how artificial intelligence (AI) and automation are revolutionizing drug development. AI is accelerating drug discovery, optimizing molecular design, and enabling predictive modeling for drug efficacy and safety. Automation is streamlining manufacturing processes, reducing costs, and ensuring consistency. Additionally, AI is enhancing personalized medicine by tailoring treatments to individual patient profiles and improving data analysis in clinical trials. This track will highlight how these technologies are transforming the pharmaceutical industry, improving efficiency, and enabling more precise and effective therapies.
AI in Drug Discovery – How AI algorithms are accelerating the identification of drug candidates, predicting molecular behavior, and optimizing drug design.
Machine Learning for Predictive Modeling – Leveraging machine learning techniques to predict drug efficacy, side effects, and patient responses.
Automation in Drug Manufacturing – The role of automation in streamlining the production process, ensuring consistency, and reducing costs.
Regulatory affairs and quality assurance are vital to ensuring the safety, efficacy, and compliance of pharmaceutical products. Regulatory affairs involve navigating complex regulatory pathways set by agencies like the FDA and EMA to obtain approval for new drugs, medical devices, and biologics. This includes preparing and submitting documentation, ensuring compliance with regulatory standards, and maintaining communication with regulatory bodies. Quality assurance focuses on ensuring that pharmaceutical products meet the highest standards of quality, safety, and consistency. It involves developing and enforcing quality control procedures, conducting inspections, and monitoring the entire production process. Both regulatory affairs and quality assurance are essential in safeguarding public health, ensuring that drugs are safe, effective, and manufactured to the highest standards.
This track delves into the science of drug action and movement within the body. Key topics include:
Pharmacokinetic Modeling – Techniques for predicting drug absorption, distribution, metabolism, and elimination.
Drug-Drug Interactions – Understanding how multiple drugs interact and affect each other’s pharmacokinetics and pharmacodynamics.
Pharmacodynamics Mechanisms – Examining how drugs exert their effects at the molecular, cellular, and system levels.
Personalized Dosing Regimens – Tailoring drug dosages based on pharmacokinetic and pharmacodynamic profiles to optimize patient outcomes.
Bioavailability and Bioequivalence – Evaluating how well a drug is absorbed and its effectiveness compared to other formulations.
Generic drug development presents several challenges, primarily revolving around ensuring bioequivalence to the original branded drug. Key hurdles include replicating the exact formulation and ensuring the same therapeutic effect while meeting regulatory standards. Variability in raw materials, manufacturing processes, and scale-up can lead to differences in quality and performance. Additionally, navigating complex patent laws, intellectual property issues, and the need for rigorous clinical testing to prove equivalence can delay the entry of generics into the market. Despite these challenges, the demand for affordable alternatives to branded medications continues to drive innovation in the generic drug industry, improving access to essential therapies worldwide.
The global pharmaceutical chemistry market is evolving rapidly, with advancements in biologics, personalized medicine, and AI-driven drug discovery leading the way. Trends include a focus on sustainable manufacturing, precision therapies, and navigating regulatory challenges. The future of pharmaceutical chemistry will see more targeted, efficient drug development, offering innovative solutions to address global health challenges and improve patient outcomes.
Advancements in Drug Discovery – How new technologies like AI, machine learning, and automation are accelerating the drug development process.
Biologics and Personalized Medicine – The growing role of biologics and precision therapies in treating complex diseases.
Regulatory and Market Access Challenges – Navigating global regulations and ensuring market access for new drugs.
Sustainable Manufacturing – Exploring greener manufacturing processes and reducing the environmental impact of drug production.
Clinical research and trials are fundamental to the development of new therapies, ensuring that drugs are safe, effective, and suitable for patient use. These trials are conducted in phases, beginning with small-scale safety tests (Phase I) and progressing to larger studies in patient populations (Phase II and III) to assess therapeutic benefits and side effects. Clinical trials are tightly regulated to protect participants and ensure reliable results, ultimately providing the evidence necessary for regulatory approval. The process is crucial for advancing medical knowledge, improving patient care, and bringing new treatments to the market.

Our webinars are thoughtfully designed to deliver value to a wide range of individuals. Whether you're looking to grow your skills, explore new opportunities or gain insights from experts, here's who will benefit most from attending:

In a world where knowledge is power, webinars have emerged as one of the most accessible and impactful ways to learn, grow and connect. Don't miss the chance to learn, grow and connect in ways that truly matter.

Registering for a webinar is essential to gain access to the unique opportunities and secure a chance to grow, connect and gain insights that can drive your personal and professional success. Webinars are more than just online meetings, they're gateways to knowledge, innovation, and growth.
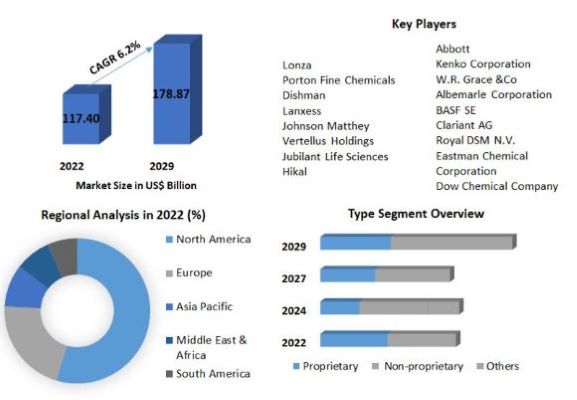
Pharmaceutical Chemicals Market: Global Industry Analysis (2023-2029)
Pharmaceutical Chemicals Market size was valued at US$ 117.40 Bn. in 2022 and the total Pharmaceutical Chemicals revenue is expected to grow by 6.2% from 2023 to 2029, reaching nearly US$ 178.87 Bn.
Pharmaceutical Chemicals Market Overview:
The pharmaceutical industry is in control of medical research, development, manufacture, and distribution. Over the last two decades, the business has grown significantly, and pharma revenues globally reached 1.3 trillion US dollars in 2022. The report explores the Pharmaceutical Chemicals market's segments (Type, Application, Industry Vertical, and Region). Data has been provided by market participants, and regions (North America, Asia Pacific, Europe, Middle East & Africa, and South America). It provides a thorough analysis of the rapid advances that are currently taking place across all industry sectors. Facts and figures, illustrations, and presentations are used to provide key data analysis for the historical period from 2017 to 2022. The report investigates the Pharmaceutical Chemicals market's drivers, limitations, prospects, and barriers. This MMR report includes investor recommendations based on a thorough examination of the Pharmaceutical Chemicals market's contemporary competitive scenario.