Welcome to the Drug Discovery 2025 Webinar, presented by GlobalMeetx. This event brings together leading Drug Discovery, researchers, industry professionals, and innovators to explore advancements, exchange ideas, and shape the future of Drug Discovery.
We are honored to host esteemed speakers and dedicated participants who are driving innovation in sustainable processes, energy efficiency, and emerging technologies. Your engagement in this event reflects a shared commitment to pushing the boundaries of Drug Discovery and addressing global challenges.
Let’s collaborate, learn, and inspire one another to create impactful solutions for a sustainable and technologically advanced future. Wishing you an insightful and productive session!


Looking to expand your knowledge and skills in pharmaceutical innovation? Our virtual Drug Discovery Webinar offers a unique opportunity to learn from leading experts, explore cutting-edge research, and connect with professionals worldwide. Gain practical insights and discover groundbreaking approaches to accelerating drug development. Join us online!
Discover a wealth of knowledge as we delve into key topics such as AI-driven drug discovery, precision medicine, novel therapeutic targets, advancements in clinical trials, and regulatory strategies for drug approval. Engage in live presentations, interactive Q&A sessions, and thought-provoking panel discussions, gaining insights that empower you to make a meaningful impact in the future of medicine.
Drug discovery is a complex and multidisciplinary process that involves identifying new therapeutic agents to treat diseases. This track explores the foundational principles, including the drug discovery pipeline, biological targets, small molecules, biologics, and high-throughput screening methods. Understanding the fundamentals sets the stage for deeper insights into modern drug development approaches.
Successful drug development begins with selecting the right molecular target. This track covers methods for identifying disease-related biological targets, validating their relevance, and assessing their druggability. Techniques such as genomics, proteomics, RNA interference, and CRISPR-based screening play a crucial role in confirming target viability.
After identifying a target, the next step is discovering potential lead compounds. This track focuses on hit identification through high-throughput screening, fragment-based drug design, and structure-based approaches. Optimization of lead compounds through medicinal chemistry, toxicity assessment, and pharmacokinetic profiling is critical to enhance efficacy and reduce side effects.
Preclinical studies evaluate the safety, efficacy, and pharmacokinetics of lead compounds before advancing to human trials. This track discusses in vitro and in vivo testing, toxicology studies, animal models, and regulatory requirements for Investigational New Drug (IND) applications. The goal is to ensure candidate drugs have a strong safety profile before clinical testing.
Optimizing how a drug is formulated and delivered can significantly impact its therapeutic success. This track explores advanced drug formulation techniques, including nanoparticles, liposomes, controlled-release systems, and biologics stabilization. A focus on enhancing bioavailability, stability, and targeted delivery ensures optimal patient outcomes.
Clinical trials are essential for evaluating the safety and efficacy of new drugs in humans. This track covers trial design, phases of clinical testing (Phase I–IV), patient recruitment, ethical considerations, and regulatory requirements. Special emphasis is placed on data collection, statistical analysis, and trial monitoring to ensure robust results.
Navigating regulatory frameworks is crucial for drug approval and market entry. This track delves into regulatory requirements from agencies like the FDA, EMA, and ICH. Topics include Good Clinical Practice (GCP), Good Manufacturing Practice (GMP), drug labeling, submission dossiers, and compliance with evolving global regulations.
Advancements in genomics and molecular profiling have led to tailored therapeutic approaches. This track examines how precision medicine uses biomarkers, companion diagnostics, and targeted therapies to enhance treatment efficacy. The role of AI, big data, and real-world evidence in personalizing drug therapies is also explored.
Developing a drug is only part of the journey—ensuring market access and commercial success is equally vital. This track discusses pricing strategies, market access barriers, reimbursement policies, health economics, and strategies for successful product launches. The role of patient advocacy and real-world evidence in market adoption is also covered.
The pharmaceutical industry is constantly evolving, with new technologies shaping the future of drug discovery. This track explores breakthroughs such as gene editing (CRISPR), synthetic biology, quantum computing, and next-generation biologics. The potential impact of AI, automation, and decentralized clinical trials is also examined.
Bridging the gap between preclinical research and patient-ready drugs requires efficient clinical development strategies. This track provides insights into adaptive trial designs, real-world evidence integration, patient-centric trials, and novel data analytics approaches to enhance clinical success rates and regulatory approvals.
Artificial intelligence and computational modeling have revolutionized drug discovery. This track covers molecular docking, virtual screening, AI-driven lead optimization, deep learning for protein structure prediction, and predictive toxicology. The integration of AI in accelerating drug development is a key focus.
The role of medicinal chemistry in refining drug candidates is critical for therapeutic success. This track explores structure-activity relationships (SAR), rational drug design, prodrug strategies, and chemical synthesis techniques to optimize potency, selectivity, and pharmacokinetics of lead compounds.
Ensuring a drug reaches its target site effectively is essential for therapeutic outcomes. This track delves into novel delivery technologies, including transdermal systems, nanocarriers, biologic drug stabilization, and injectable sustained-release formulations. Strategies to overcome solubility, permeability, and stability challenges are highlighted.
Transitioning from lab-scale production to commercial manufacturing requires robust strategies. This track covers Good Manufacturing Practices (GMP), process optimization, quality control, and regulatory compliance in large-scale drug production. Additionally, supply chain logistics, raw material sourcing, and risk management strategies are explored to ensure global drug availability.

Our webinars are thoughtfully designed to deliver value to a wide range of individuals. Whether you're looking to grow your skills, explore new opportunities or gain insights from experts, here's who will benefit most from attending:

In a world where knowledge is power, webinars have emerged as one of the most accessible and impactful ways to learn, grow and connect. Don't miss the chance to learn, grow and connect in ways that truly matter.

Registering for a webinar is essential to gain access to the unique opportunities and secure a chance to grow, connect and gain insights that can drive your personal and professional success. Webinars are more than just online meetings, they're gateways to knowledge, innovation, and growth.
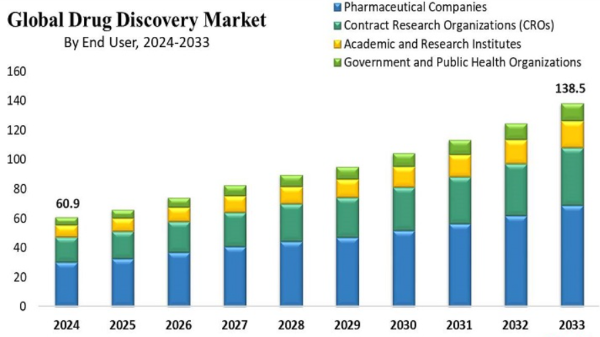
The Drug Discovery Market size is estimated at USD 106.70 billion in 2025, and is expected to reach USD 146.80 billion by 2030, at a CAGR of 6.59% during the forecast period (2025-2030).Amid the COVID-19 pandemic, the health systems of various countries rapidly invested in research and development to combat the virus. Potential compounds were screened from the CHEMBL, ZINC, FDA-approved drugs, and molecules under clinical trials. Research groups worldwide have identified drugs for the treatment of COVID-19 by screening both novel and existing drugs for their ability to alleviate symptoms and stop viral replication. Thus, the drug discovery market was positively impacted due to the urge to find a cure for COVID-19. Furthermore, post the pandemic, there is a rise in investments in different modalities of drug development, such as gene and cell therapies for both COVID-19 diseases and other novel diseases, which is expected to impact the market's growth as per the analysis positively. Furthermore, the research for developing antiviral drugs for COVID-19 is still in progress. For instance, in May 2022, the National Institute of Allergy and Infectious Diseases (NIAID) awarded approximately USD 577 million to establish nine Antiviral Drug Discovery (AViDD) Centers for Pathogens of Pandemic Concern. The AViDD centers will carry out creative, interdisciplinary research to create candidate COVID-19 antivirals, particularly those that can be used outside of a hospital setting, as well as antivirals that target particular viral families with a high potential to trigger a pandemic in the future. Such grants are expected to drive the drug discovery market's growth post-pandemic.